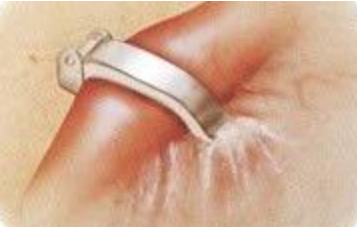
Filshie Clips
The medical procedure involves the use of titanium clips coated with silicone rubber to encircle each fallopian tube. The principle behind the method is simple: by applying continuous pressure, the clips obstruct the fallopian tubes, preventing eggs from reaching sperm and thereby preventing pregnancy.
Filshie Clips have a tendency to migrate after implantation. Clips have been found in the liver, uterus, bowels, abdominal wall, and other organs/pathways in the abdominal/pelvic region. Reports suggest that migration occurs in over 25% of cases, potentially leading to serious complications that often require surgical intervention to remove the displaced clips.
Studies
A 2001 review by the inventor, Marcus Filshie, published in Reviews of Gynecological Practice conveys his own findings of a 25% device migration rate post implant.
MDL
No current MDL being sought
Litigation Updates
Texas firm, Griffin Purnell, has been leading the charge filing cases in some tough jurisdictions, surviving MTD in February 2023 in District of N. GA on almost all causes of action. January 2023 another case survived MTD in the District of Middle Alabama. In March 2024 plaintiffs survived MTD by sufficiently pleading parallel claims in the District of Rhode Island.
Important Links
- https://www.sciencedirect.com/science/article/abs/pii/S1471769701800228
- https://onlinelibrary.wiley.com/doi/10.1155/2020/4809859
- https://casereports.bmj.com/content/16/6/e256013
MDL Link
No current MDL