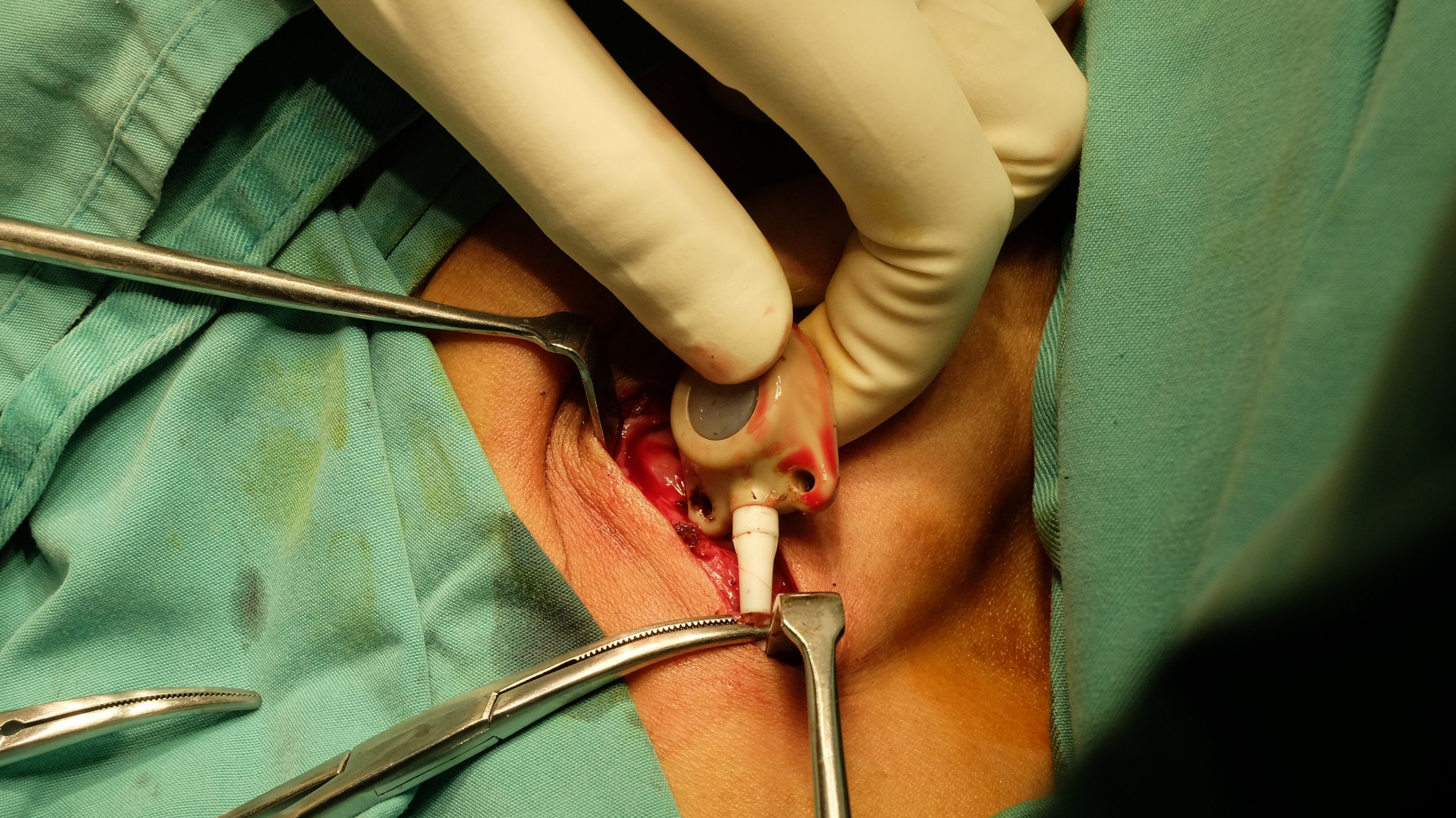
Bard PowerPort®
Originally approved by the FDA in 2000, the Bard PowerPort was designed as an implantable port for easy, long-term access to administer chemotherapy, medication, fluids, and nutrition directly into the bloodstream. However, since its introduction, numerous patients have reported complications with the device, including fractures and migration of the catheter. These issues have been linked to serious health risks including infections, blood clots, punctures of the heart, fragments becoming trapped in either the heart or lungs, and other life-threatening conditions like septic infections.
While the initial concept of the Totally Implantable Venous Access Device (TIVAD) was first approved in 1981, numerous variations of the device have since been approved since the original PMA. All of the devices sold today have been approved via the 510(k) fast track process by the FDA. Becton Dickinson’s (NYSE: BDX) Bard subsidiary maintains roughly 80% market share for sales of these devices in the United States with their PowerPort® being the flagship product.
Medical Research
A peer reviewed meta analysis published in 2021, which encompassed more than 93,000 patients with an insitu time of up to five years revealed an alarmingly high complication rate of 59%. The same study identified infections in nearly 18% of patients, thrombovalscular events (blood clots) occurred in 37%, and mechanical complications (including catheter fracture and migration) occurred in more than 10% of patients implanted with these devices. This study was extraordinarily important as it was the first study to look at extended insitu values. Considering manufacturer reps and medical professionals now recommend leaving these devices in for years, and in some instances, in perpetuity, the relevance of these findings are paramount. The size of the patient population of this study support the veracity and confidence levels of the conclusions derived from the data.
A design defect during the manufacturing process regarding the distribution of the radiopaque agent, barium sulfate, in the catheter tube was first identified and conveyed to manufacturers in 1985. These findings were extrapolated on via studies published in 2014 and 2017. This research was then perfectly synthesized in a Letter to the Editor by one of the most highly acclaimed researchers on the topic, which helped define the mechanism for causation of the fracture.
Manufacturers including those producing and selling TIVAD devices had utilized the now defunct Alternative Summary Reporting ‘self reporting’ program (ASR) from 1997 through April 2019, when it was shuttered after a scathing exposé in Kaiser Health News showed how manufacturers were hiding issues not only from the public, but also from medical professionals by only reporting Device Experience Network (DEN’s), and not reporting the same issues as Medical Device Reports (MDR’s) on the public MAUDE database. An example of this egregious practice was that from 2004-2010 there were 0 MDR’s reported to MAUDE by port-a-cath manufactures, while there were over 3,600 DEN’s filed in the ASR. From 2004-2017 there were more than 9,000 DEN’s submitted, while only 1,200 showed up in MAUDE. This lack of transparency likely led to a false sense of safety and security by medical professionals during the rapid adoption and utilization of these devices over the past two decades.
MDL
MDL #3081, District of Arizona, Judge David G. Campbell
Current Status Of Litigation
The Bard PowerPort (MDL #3081) is one of the newer mass torts that has developed over the past four years. US District Court of Arizona Judge David G. Campbell was selected by the JPML panel in August 2023 to oversee coordinated discovery and pretrial motions.
Judge Campbell, who previously presided over Bard IVC Filters MDL #2641, set an April 1, 2024 deadline for the bellwether pool. Currently there are more than 260 cases on file, with thousands more expected in the coming year. For context, Legal Marketing Concepts has retained more than 4,500 claimants on behalf of our law firm partners.
A group of 48 (24 plaintiff/24 defense) cases will be selected from those for the PFS/DFS Group #1 and must be submitted to the court by July 1, 2024. On or by December 10, 2024 counsel will exchange 5 picks each from this pool and meet-and-confer on another 5 picks to be submitted as Discovery Group #1. The court will pick from those the following week, with plaintiff and treater depositions to follow.
April 5, 2025 is the date the court has set to select Bellwether Group #1, which will be comprised of 3 plaintiff and 3 defense picks, with expert disclosures to be submitted shortly thereafter. Though it has yet to be calendared, the Daubert briefing schedule is anticipated for December 2025 and plaintiffs are ready with all of their experts already lined up.
Judge Campbell has been adamant about pushing this litigation forward quickly (denying discovery delay requests by defense), wrapping the bellwethers by the end of 2026 and seeing the litigation resolved by the end of 2027. In early status conferences he envisioned trying the bellwethers two months apart, which will likely now push into the end of 2026 if all six move forward as planned.
Important Links
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8077681/
- https://pubmed.ncbi.nlm.nih.gov/4055822/
- https://pubmed.ncbi.nlm.nih.gov/27824502/
- https://www.sciencedirect.com/science/article/abs/pii/S1751616116302582
- https://www.ajronline.org/doi/full/10.2214/AJR.17.18125
- https://kffhealthnews.org/news/hidden-fda-database-medical-device-injuries-malfunctions/
MDL Link
Bard PowerPort MDL #3081
Case Management Orders Links
- CMO 1
- CMO 2
- CMO 3
- CMO 4
- CMO 4 Ex.1
- CMO 4 Ex. 2
- CMO 4 Ex. 3
- CMO 5
- CMO 6
- CMO 7
- CMO 8
- CMO 9
- CMO 10
- CMO 10 (Amended)
- CMO 11
- CMO 12
- CMO 13
- CMO 14
- CMO 15
- CMO 16
- CMO 17
- CMO 18
- CMO 19
- CMO 20
- CMO 21
- CMO 22
- CMO 23
- CMO 24
- CMO 25
- CMO 26
- CMO 27
- CMO 28
- CMO 29
- CMO 30
- CMO 31
- CMO 32